United States Prescription Prices Vs. The World Market
Does Nationalizing Health Care Help Individuals? What Can The Future Look Like?
If America Forces Pharma To Bring Prices Comparable To The International Socialized Health Care Countries- What Will The Final Price Be?
On May 12, 2025, President Donald Trump signed an executive order titled: “Delivering Most-Favored-Nation Prescription Drug Pricing to American Patients.” This policy aims to align U.S. prescription drug prices with the lowest prices paid by other developed nations, addressing concerns that Americans are subsidizing global pharmaceutical costs.
Key Provisions of the Executive Order
Price Alignment: Directs the Department of Health and Human Services (HHS) to negotiate drug prices to match the lowest prices paid by comparable countries.
Scope: Applies to drugs covered under Medicare and Medicaid, with potential expansion to the private market.
Enforcement: If pharmaceutical companies fail to comply, the administration may pursue regulatory actions, including facilitating drug importation from countries with lower prices.
Trade Measures: Empowers the U.S. Trade Representative and Secretary of Commerce to address foreign practices that contribute to higher U.S. drug prices.HHS.gov+5The White House+5The White House+5
Clearly, forcing our way into the “International Buyers Warehouse” will improve the prices Americans pay for prescription medications. Understanding that we in the United States have provided the legislative protections that have promoted pharmaceutical monopolies, it stands apparent that we too, pay the highest prices- specifically because of that arrangement. After decades of allowing the rest of the world to arbitrage this market favoritism, President Trump is calling this out into the open and demanding a realignment.
If followed to its logical conclusion, taking this stand will drive prices of medications up on socialized healthcare systems (Canada, United Kingdom, etc) and place a relief valve on United States Price pressures. The individuals in the socialized nations will see cost harm and the US market will see cost reductions. The outcome will be more “equitable” en large but there will be plenty of squealing along the way. If this process goes to full international access (likely due to Pharma’s obstinance), things are going to look very different. I hope pharma drags its feet and we put the fire on full heat.
How Did We Come To This Market Situation?
The Hidden Engine: Patents and Profit Protection
At the core of pharmaceutical pricing lies patent protection. Drug companies receive 20-year patents to protect their discoveries, which block generic competition. These patents are often extended through a practice known as "evergreening" — filing additional patents for minor modifications to maintain exclusivity. Take the Glp1 agonist injection devices as an example.
This global patent regime is enforced by the TRIPS Agreement, a treaty established in 1995 under the World Trade Organization (WTO). It standardizes International Patent protections worldwide, including for medicines, and pressures countries — even low-income ones — to adopt the same patent rules.
This system rewards innovation but also delays access to affordable generics, especially in countries that previously produced low-cost versions of brand-name drugs.
The TRIPS Agreement — short for Trade-Related Aspects of Intellectual Property Rights — is the most comprehensive international agreement on intellectual property (IP) and has had a profound impact on the global pharmaceutical landscape.
TRIPS is a binding international treaty that sets minimum standards for intellectual property protection (including patents, copyrights, trademarks, etc.) among World Trade Organization (WTO) member countries.
It came into force in 1995 as part of the founding of the WTO.
Historical Context: Why TRIPS Was Created
📉 Before TRIPS:
Many countries did not offer patent protection for pharmaceuticals or only for production methods (not drug products).
This allowed generic manufacturing and cheaper drug access, particularly in countries like India and Brazil.
💼 Enter the U.S. and Big Pharma:
During the 1980s, U.S. corporations — especially pharmaceutical, software, and entertainment industries — lobbied for stronger international IP protections.
They argued weak IP laws abroad led to "piracy" and revenue loss.
The U.S. tied IP enforcement to trade policy (via Special 301 Reports) and pushed for IP to be part of multilateral trade negotiations.
🌐 Uruguay Round (1986–1994):
The TRIPS Agreement was negotiated as part of the Uruguay Round of the General Agreement on Tariffs and Trade (GATT).
TRIPS became a condition for joining the WTO, pressuring countries to accept IP protections in exchange for global market access.
The history of Pharma regulation is more involved but I think the above summaries provide adequate perspective. Add the following:
Why U.S. Prices Are Higher
The U.S. does not regulate drug prices at the federal level.
The U.S. allows direct-to-consumer advertising and longer exclusivity periods via FDA rules and patent law interplay (e.g., biologics get 12 years exclusivity).
Other countries often:
Use reference pricing or price negotiations.
Wait for generics or biosimilars (more delayed where patents are tight).
Certainly. While TRIPS standardizes minimum patent terms internationally (20 years), governments differ widely in how they grant exclusivity beyond the patent—especially for biologics and new chemical entities (NCEs). These regulatory exclusivities are critical to drug pricing and generic/biosimilar competition.
Based Upon The Protection Favoritism For Pharma In The US, The Return On Investment Proposition Uniquely Places Costs On Our Market
Key Stat
A 2020 study published in Health Affairs showed that U.S. prices account for 78% of total global pharmaceutical profits, while the rest of the world combined contributes only 22%.
As the public is likely aware, Secretary of Health and Human Services, Robert Kennedy Jr. has floated the idea of removing direct to consumer advertising for pharma. Here is some interesting information to have in mind. With the exception of Wegovy, the products in this list of top 5 Direct To Consumer spenders are for biologic products. Biological patent rules in the US vs the world is a topic for another post.
More contemporary updates on how marketing dollars are spent, indicate it has dramatically shifted away from physician directed advertising to direct to consumer advertising.
Updated Breakdown of U.S. Pharmaceutical Advertising (2023–2024)
Total Advertising & Promotion (A&P) Spend (2023): $13.8 billion by the top 10 pharmaceutical companies. Fierce Pharma+3Informa TechTarget+3AHIP+3
Direct-to-Consumer (DTC) Advertising:
Total DTC Spend (2023): Approximately $13.8 billion.
Television Advertising: $5.15 billion in national TV ads.
Digital Advertising: $7.76 billion on display ads. AHIP+2CSRxP+2Informa TechTarget+2Marketing & PharmaEMARKETER
Physician-Directed Marketing:
While specific figures for 2023 are limited, historical data suggests that physician-directed marketing (including free samples, sales representatives, and medical journal advertising) remains substantial but may be outpaced by DTC spending.
Total pharma marketing spent in the US exceeds research and development costs. Big pharma has always whined about the cost of R and D as the reason prices have to be so high in the United States. Look at what they do, not what they say.
What Would An International Prescription Drug Market Look Like?
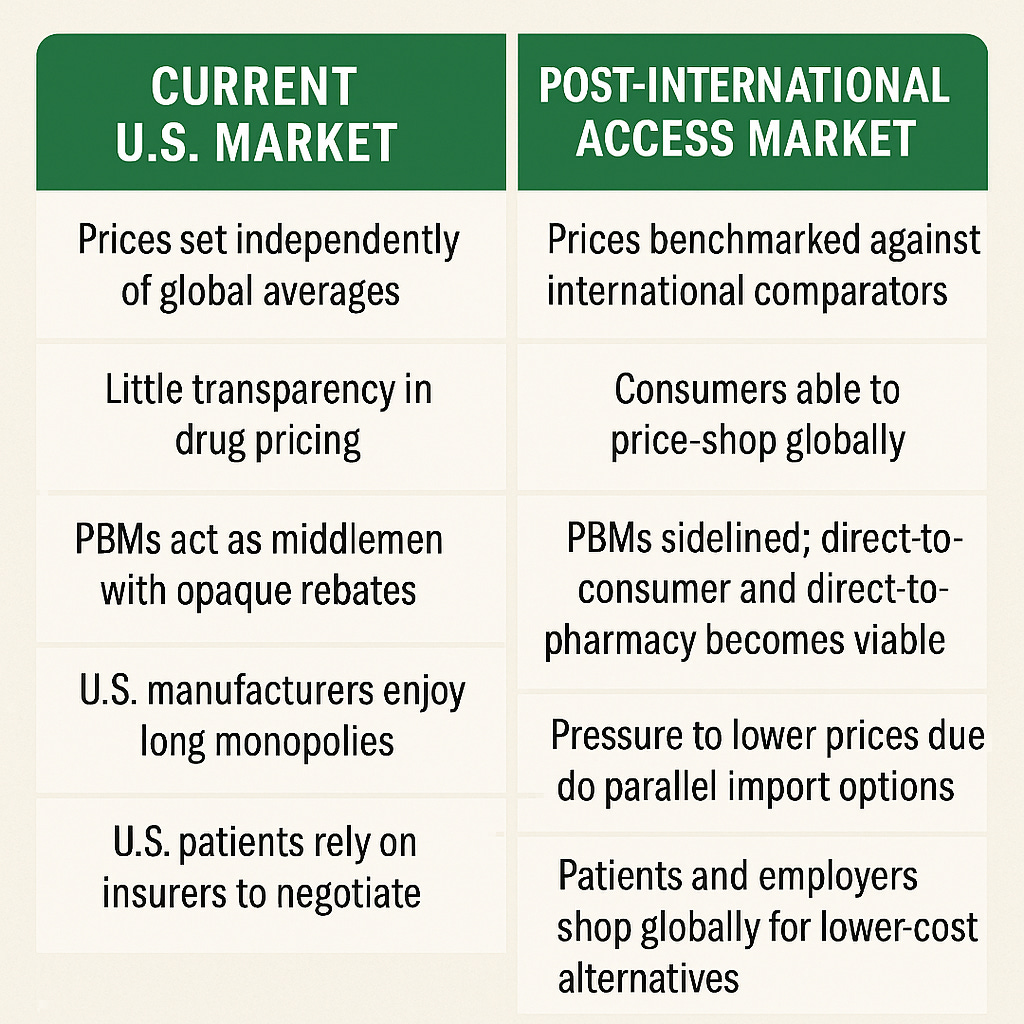
Allowing Americans to shop internationally for prescription medications would significantly disrupt the current U.S. pharmaceutical market by introducing competition, undermining price protectionism, and shifting power away from pharmacy benefit managers (PBMs) and domestic monopolies. Here's a structured look at what such a market would look like and what currently exists:
Is there any excuse in this modern day society for this not to happen? The only reason is isn’t happening is special interests lobbies have pirated the market using government laws stacking the deck in big pharma and big insurance’s favor. An opaque market allows for the “legal theft” from market efficiency. Note PBM’s are literally sidelined if we allow international access.
AND FINALLY:
Does an International Pharma Market Already Exist?
Yes — in both wholesale and retail forms:
🔹 Wholesale Markets
Countries with reference pricing often re-export medications to lower-cost nations (the EU allows this within its borders).
Major wholesalers exist in:
Germany (parallel trade to the UK, France)
India (generic manufacturing hub)
Turkey, Israel (regional supply chains)
🔹 Retail Access
Consumers already access drugs internationally via:
Online pharmacies (Canada, UK, India)
Medical tourism (Mexico, Thailand, India)
However, U.S. law currently prohibits personal importation except under FDA discretion (usually for 90-day supplies of non-controlled substances for personal use).